The Impact of Gonadotropin-Releasing Hormone Analogues and Subsequent Cross-Sex Hormones on Bone Health: A Systemic Review
This section summarises the literature on the impacts of puberty blockers on bone health with a systemic review. Please cite as: Tegg, S., The Impact of Gonadotropin-Releasing Hormone Analogues and Subsequent Cross-Sex Hormones on Bone Health: A Systemic Review. Fully Informed. 2021.
Abstract
Methods: A literature search of the Pubmed database for studies reporting bone mineral density (BMD) changes in adolescents receiving GnRHa and subsequent cross-sex hormones (CSH), or GnRHa and follow-up after treatment withdrawl was conducted. A random effects meta-regression was used to compute changes to BMD and BMD z-score (BMD-Z) across included studies. Pairwise T-tests of cohort level results were used to compare follow-up BMD results with baseline, CSH start, and healthy population references.
Results: Six cohort studies reporting changes in BMD while on GnRHa treatment for gender dysphoria. Four of the studies reported BMD changes under subsequent testosterone treatment in females, and three reported BMD changes of males under subsequent estrogen treatment. The total number of participants were 371 for GnRHa, 144 for testosterone, and 58 for estrogen. No studies examined BMD recovery or otherwise after GnRHa was stopped and endogenous sex hormone production began. In the random effects model, the BMD-Z of GnRHa-treated adolescents declined -0.83 standard deviations (SD) (p <.001). BMD-Z increased 0.53 SD under subsequent testosterone treatment and was lower than baseline by -0.22 SD, but this was not significant (p = .12). Under subsequent estrogen treatment BMD-Z increased 0.25 SD but this was not significant, and follow-up values were -0.29 SD below baseline values (p = .08).
Conclusion: Under GnRHa treatment adolescent BMD stabilises or declines slightly during a period when it is expected to increase. Consequently, BMD values compared to age-matched peers decreases. Subsequent testosterone treatment may recover BMD status in female subjects, but the evidence is mixed for recovery in male subjects treated with estrogen, and lacking for subjects who withdraw from hormone treatments. Patients and their parents should be advised of impacts to BMD and that the impacts may be long-term.
Introduction and Background
GnRha treatment induces a state of suspended sex hormone production and pubertal development (hypogonadotropic hypogonadism) . Clinicians treating adolescents with GnRHa (also known as “puberty blockers”) propose that the treatment relieves distress associated with undesired pubertal development and will allow the adolescent’s adult body to more closely resemble their identified sex.
For patients for whom gender dysphoria persists, CSH hormone treatment usually begins at age 16. CSH treatment directly changes some of the secondary sex characteristics to align with the opposite sex (body hair, fat distribution, vocal register) and can be permanent ( Oliphant et al., 2018 ; Hembree et al., 2017 ).
In NZ, GnRHa treatment may continue through to age 20 ( Oliphant, 2017 ). It is unclear how common the continuation of GnRHa treatment past age 16 is in other countries or whether this is a NZ-specific practice.
On March 1st, 2021, two Otago University academics, Dr Sue Bagshaw and Dr Jane Spittlehouse released a statement on the results of and unpublished student’s summer 2020/2021 literature review. The literature review concerned the impacts and benefits of GnRHa for treating adolescents with gender dysphoria. In that statement, Bagshaw and Spittlehouse make claims regarding the impacts of GnRHa and subsequent cross-sex hormones (CSH), or treatment withdrawal on the bone health of adolescents.
The claims were paraphrased in a media article ( Broughton, 2021 ):”One exception is that use of puberty blockers does result in a decrease in bone mineral density, but this normalises when GnRH analogues are stopped and either a return to self-produced or cross-sex hormones are started.”
”Bagshaw said reduced bone density was similar to the effect of the depo provera contraceptive injection, and was reversible.”
In another article, similar claims were made by Dr Rachel Johnson (Chisholm, 2021):
The position above is distinct from that of (a) clinicians at the The Royal Children’s Hospital in Melbourne, Australia who state that under subsequent CSH, BMD “ may not reach the bone density levels of age and birth-assigned sex-matched controls. ” ( Notini et al., 2020, p. 5 )“Bone health is one of the areas we particularly focus on because we know that blockers do affect bone development. Research is showing that when you either stop the blocker or add in gender-affirming hormones then the bone density improves. Bone density is restored within approximately three years.”
Or (b) clinicians at the Mayo Clinic in Rochester MN, USA “ With initiation of [CSH], Z-scores improve but may not return to pretreatment Z-scores. ” ( Davidge-Pitts and Clarke, 2019, p. 40 )
Further, and confusingly, a secondary statement summarising the same literature review makes a contradictory claim regarding the recovery of BMD for those who do not proceed to CSH ( Spittlehouse et al., 2021 ):
“The research indicates that there is a decrease in bone mineral density but that appears to normalise after puberty blockers are stopped and the new sex hormones are commenced (for those who choose to transition). There is a lack of evidence around the impacts of bone mineral density for those who do not receive new sex hormones. ” (emphasis added)This article examines the accuracy of the above claims and attempts to clarify the apparent contradictions. GnRHa and CSH are controversial treatments. Puberty is an important period for bone mass accumulation. Adolescents who enter puberty at older ages have persistently lower BMD than peers ( Elhakeem et al, 2019 ). An induced pubertal blockade could therefore be expected to have similar effects. An adolescent patient treated with GnRHa has reported a high fracture rate ( Bannerman, 2019 ). A second case study reports an adolescent with BMD -2 SD below the mean after 3 years of GnRHa treatment ( Pang et al., 2020 ). A third case of long-term untreated hypergonadotropic hypogonadism has reported osteoporosis and vertebral fractures in middle age ( Carsote et al., 2016 ).
In adults, CSH hormones increase BMD in the short-term ( Wiepjes et al., 2017 ), but estrogen treatment in males appears to produce a slight decrease in BMD in the long-term ( Delgado-Ruiz et al., 2019 ). However, it is not clear the extent to which lower levels of physical activity in the transgender male population confound these results.
The NHS has recently revised its advice on GnRHa ( Kirkup, 2020 ). Following an ethical review, the Karolinska Institute no longer offers GnRHa or CSH to patients under 16 years of age, citing the risk of osteoporosis in later life (amongst other risks) ( SEGM, 2021 ). Patients, and their parents have a legal right to clear and accurate information on the impacts of any treatment under consideration.
Clinical decision-making is not served by the polarised debate, nor the contradictory statements, that have emerged around this issue. A systematic review can help clarify the likelihood and magnitude of any bone health impacts.
Absolute vs Relative Changes in Bone Mineral Density
A potential source of confusion regarding adolescent bone health is the distinction between absolute changes in BMD versus changes in BMD relative to a healthy population reference. During child-hood, BMD increases until peak bone mass is reached in early adulthood. Puberty is the peak period for BMD accumulation (Figure 1.). According to Boot et al. (1997) puberty stage is the major independent determinant of BMD in girls . Stable absolute BMD in adolescence therefore represents a failure to accumulate adult BMD levels, and a decline in BMD relative to a healthy population reference.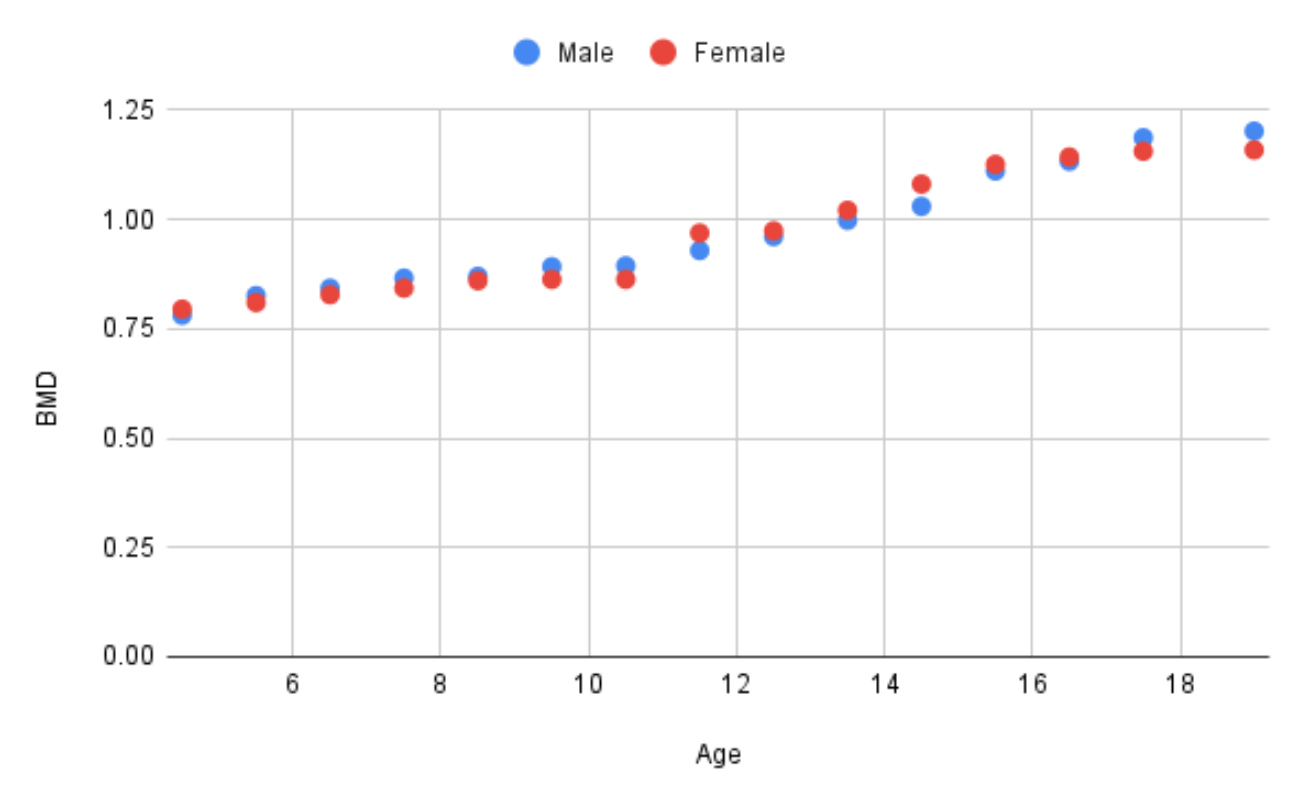
Therefore reported changes to absolute BMD can obscure adolescent bone health status, while relative measures provide a clearer picture of adolescent bone health under treatment ( Klink et al., 2015 ).
BMD reference population comparisons are measured with Z-scores. A Z-score of 0 represents the mean (50th percentile) for that population. A Z-score of -1 represents BMD 1 standard deviation (SD) below the mean, or approximately the 16th percentile. In adults, a BMD Z-score between -1 and -2.5 is considered to be osteopenia, and below -2.5 (0.6th percentile) is considered osteoporosis. However, adolescent BMD Z-scores are interpreted differently. A Z-score below -2 is considered “low for age” and a diagnosis of paediatric osteoporosis relies on fracture history in addition to z-scores ( Bachrach & Gordon, 2016 ).
Method
Literature search
The search strategy included articles that: (a) were intervention studies reporting absolute BMD or BMD-Z measures, (b) of gender dysphoric adolescents, (c) treated with puberty blockers, or both puberty blockers and subsequent cross-sex-hormones, (d) and written in English.
The search strategy excluded articles that were systemic reviews, editorials, opinion pieces, and articles on the treatment of central precocious puberty (CPP) with GnRHa. Children with CPP usually start GnRHa treatment at age ~8 and discontinue at age ~12 ( Poomthavorn et al., 2011 ). Gender dysphoric adolescents usually start treatment at age 11-15 and discontinue at age ~16 ( Joseph et al., 2019 ; Klink et al., 2015 ; Schagen et al., 2020 ). Only GnRHa treated gender dysphoric adolescents experience a condition analogous to “delayed puberty”. GnRHa-treated children with CPP do not appear to suffer BMD impacts ( Guaraldi et al., 2016 ). The current study considers two groups separate for the purposes of assessing BMD impacts with distinct potential outcomes.
The PubMed database was searched with the following search strategy: [GD] defines a variable term with variations: “gender dysphoria”, “gender identity”, and “transgender”. [puberty] defines a variable term with variations: “puberty” and “adolescent”. [CSH] defines a variable term with variations: “hormone”, “testosterone” and “estrogen”. Searches combined term variations into 15 searches using the following blueprint:
- GnRHa AND bone AND [puberty] AND [GD] NOT precocious NOT CPP
- [CSH] AND bone AND [GD]
Data analysis
The R package mixmeta was used to create a random effects meta-regression of treatment impacts on BMD-Z. The main model compared baseline and follow-up values. A secondary model adjusted for treatment duration. Data and analysis code are made available on a google sheet and repository .Results
The literature search resulted in 111 articles. Results were screened using the above inclusion criteria and narrowed the results to the six studies ( Carmichael et al., 2021 ; Joseph et al., 2019 ; Klink et al., 2015 ; Schagen et al., 2020 ; Stoffers et al., 2019 ; Vlot et al., 2017 ). Table 1. presents the studies with a breakdown by participant cohort. Study cohorts are listed by first author, year of publication, treatment, number of participants at baseline, sex, mean age of particpants at the start of treatment, and treatment duration at the final reported follow-up.Table 1. Study cohorts characteristics
| Study | Year | Treatment | n (start) | Sex | Mean age (start) | Duration (months) |
|---|---|---|---|---|---|---|
| Carmichael | 2021 | GnRHa | 44 | M+F | 13.8 | 36 |
| Schagen | 2020 | GnRHa | 51 | M | 14.1 | 36 |
| Schagen | 2020 | GnRHa | 70 | F | 14.5 | 36 |
| Joseph | 2019 | GnRHa | 31 | M | 13.2 | 31 |
| Joseph | 2019 | GnRHa | 39 | F | 12.6 | 34 |
| Stoffers | 2019 | GnRHa | 62 | F | 16.5 | 8 |
| Klink | 2015 | GnRHa | 12 | M | 14.9 | 20 |
| Klink | 2015 | GnRHa | 19 | F | 15.0 | 17 |
| Vlot | 2017 | GnRHa | 33 | F | 15.1 | 14 |
| Vlot | 2017 | GnRHa | 22 | M | 13.5 | 30 |
| Schagen | 2020 | Testosterone | 42 | F | 16.9 | 36 |
| Stoffers | 2019 | Testosterone | 62 | F | 17.2 | 12 |
| Vlot | 2017 | Testosterone | 33 | F | 16.3 | 24 |
| Klink | 2015 | Testosterone | 19 | F | 16.4 | 66 |
| Schagen | 2020 | Estrogen | 36 | M | 16.2 | 36 |
| Vlot | 2017 | Estrogen | 22 | M | 16.0 | 24 |
| Klink | 2015 | Estrogen | 12 | M | 16.2 | 66 |
Vlot et al. (2017) and Klink et al. (2015) were conducted by the same research team and 12 subjects overlap. No studies tracked BMD after a withdrawal of GnRHa and subsequent endogenous sex hormone production re-started.
Data extraction
BMD location, mean absolute BMD, and mean Z-score, sex and age of subjects at the treatment intervals including baseline, the number of subjects, the treatment type, treatment length, dosage, and variation measures were extracted. Treatments did not vary by dosage regime and was consequently discarded as a unit of interest.
Where the standard error was unavailable, standard deviation and confidence intervals were converted into standard errors using the appropriate formulas. Table 2. summarises the results of GnRHa treatment on absolute BMD and BMD-Z. A tilde (~) indicates the study found no significant differences. Down (↓) and up (↑) arrows indicate significant decreases or increases respectively. The number of arrows represents the significance threshold met by the reported P value:
| Sign | P value |
|---|---|
| ↓ / ↑ | ≤ 0.05 |
| ↓↓ / ↑↑ | ≤ 0.01 |
| ↓↓↓ / ↑↑↑ | ≤ 0.001 |
To enhance table’s readability, symbols have been colour-coded blue (neutral) or red (negative) to indicate the impact on bone health depending on whether the measure is absolute or relative.
Table 2. Impacts of GnRHa treatment on BMD and BMD Z-Score
| Study | Sex | Measure | n | BMD | BMD-Z |
|---|---|---|---|---|---|
| Klink | M | Spine BMAD | 11/12 | ~ | ~ |
| Klink | M | Spine aBMD | 12/11 | ~ | ~ |
| Klink | M | Hip BMAD | 12/10 | ~ | ~ |
| Klink | M | Hip aBMD | 14/6 | ~ | ~ |
| Klink | F | Spine BMAD | 18 | ~ | ↓↓ |
| Klink | F | Spine aBMD | 18 | ↓↓ | ↓↓↓ |
| Klink | F | Hip BMAD | 18 | ~ | ~ |
| Klink | F | Hip aBMD | 18 | ↓↓ | ↓↓↓ |
| Vlot | F | Hip BMAD | 10 | ~ | ~ |
| Vlot | F | Hip BMAD | 23 | ↓↓ | ↓↓ |
| Vlot | F | Spine BMAD | 11 | ~ | ↓↓ |
| Vlot | F | Spine BMAD | 23 | ↓↓ | ↓↓ |
| Vlot | M | Hip BMAD | 16 | ~ | ~ |
| Vlot | M | Hip BMAD | 6 | ~ | ~ |
| Vlot | M | Spine BMAD | 15 | ~ | ↓↓ |
| Vlot | M | Spine BMAD | 5 | ~ | ~ |
| Carmichael | M+F | Spine BMD | 12 | ↑ | ↓? |
| Carmichael | M+F | Hip BMD | 12 | ~ | ↓? |
| Stoffers | F | Spine BMD | 62 | ↓↓↓ | ↓↓↓ |
| Stoffers | F | L Hip BMD | 62 | ↓↓↓ | ↓↓↓ |
| Stoffers | F | R Hip BMD | 62 | ↓↓↓ | ↓↓↓ |
| Schagen | M | Spine aBMD | 15 | ↑ | ↓ |
| Schagen | M | Spine aBMD | 36 | ↑ | ↓ |
| Schagen | M | Hip aBMD | 15 | ↑ | ↓ |
| Schagen | M | Hip aBMD | 36 | ~ | ↓ |
| Schagen | M | Body BMD | 15 | ↑ | ↓ |
| Schagen | M | Body BMD | 36 | ~ | ↓ |
| Schagen | M | Spine BMAD | 15 | ~ | ↓ |
| Schagen | M | Spine BMAD | 36 | ~ | ↓ |
| Schagen | M | Hip BMAD | 15 | ~ | ~ |
| Schagen | M | Hip BMAD | 36 | ↓ | ↓ |
| Schagen | F | Spine aBMD | 14 | ↑ | ↓ |
| Schagen | F | Spine aBMD | 56 | ↓ | ↓ |
| Schagen | F | Hip aBMD | 14 | ↑ | ↓ |
| Schagen | F | Hip aBMD | 56 | ↓ | ↓ |
| Schagen | F | Body BMD | 14 | ↑ | ↓ |
| Schagen | F | Body BMD | 56 | ↓ | ↓ |
| Schagen | F | Spine BMAD | 14 | ~ | ↓ |
| Schagen | F | Spine BMAD | 56 | ↓ | ↓ |
| Schagen | M | Hip BMAD | 14 | ↓ | ↓ |
| Schagen | M | Hip BMAD | 56 | ↓ | ↓ |
| Joseph | M | Hip BMD | 10 | ~ | ↓↓ |
| Joseph | M | Spine BMD | 10 | ~ | ↓↓↓ |
| Joseph | M | Spine BMAD | 10 | ~ | ↓↓↓ |
| Joseph | F | Hip BMD | 21 | ~ | ↓↓↓ |
| Joseph | F | Spine BMD | 21 | ~ | ↓↓↓ |
| Joseph | F | Spine BMAD | 21 | ~ | ↓↓↓ |
All cohort-measures with at least 21 participants reported a decrease in BMD Z-score. The main random effects model compared baseline and follow-up periods irrespective of treatment duration. The modelled change in BMD-Z across all studies was -0.83 (95% confidence interval -0.65 – -1.00, P <.001). The model predicts a mean BMD-Z of -0.24 at baseline and -1.06 after GnRHa treatment. The model was adjusted for sex but this was not significant. A secondary random effects model of the followup periods modeled the impact of treatment duration. An additional month of GnRHa treatment was correlated with an -.04 decline in BMD-Z but the P value (0.0827) did not reach the usual threshold for statistical significance.
Cross Sex Hormones
Table 3. compares BMD-Z under subsequent CSH with baseline (i.e. the start of GnRHa treatment), start of CSH treatment, and a reference population. Comparisons to the start of CSH treatment are those reported by the studies. Klink et al. (2015) also reports comparisons to baseline values. In all other baseline and reference cases a pairwise T-test is computed.Table 3. Differences between BMD z-scores, baseline, start of testosterone treatment, and reference population values.
| Study | Measure | n | vs Baseline | vs Start | vs Reference |
|---|---|---|---|---|---|
| Schagen | spine aBMD | 5 | - | ↑ | - |
| Schagen | hip aBMD | 5 | - | ↑ | - |
| Schagen | body aBMD | 5 | - | ↑ | - |
| Schagen | spine BMAD | 5 | - | ↑ | - |
| Schagen | hip BMAD | 5 | - | ↑ | - |
| Schagen | spine aBMD | 37 | ↓↓ | ↑ | - |
| Schagen | hip aBMD | 37 | - | ↑ | - |
| Schagen | body aBMD | 37 | ↓↓ | ↑ | - |
| Schagen | spine BMAD | 37 | - | ↑ | - |
| Schagen | hip BMAD | 37 | - | ~ | - |
| Stoffers | spine BMD | 15 | ↓ | ~ | - |
| Stoffers | L hip BMD | 15 | ↓ | ↑↑ | - |
| Stoffers | L hip BMD | 15 | ↓ | ↑ | - |
| Vlot | hip BMAD | 10 | - | ↑↑ | - |
| Vlot | spine BMAD | 11 | - | ↑↑ | - |
| Vlot | hip BMAD | 23 | - | ↑ | - |
| Vlot | hip BMAD | 23 | - | ↑↑ | - |
| Klink | spine BMAD | 19 | - | ↑↑ | - |
| Klink | hip BMAD | 19 | ↓ | ~ | - |
| Klink | hip aBMD | 16 | - | ~ | - |
BMD-Z increased under testosterone treatment in most cohort-measures and followup values were not significantly different from the reference population. In 6 out of 20 cohort-measures BMD-Z after testosterone treatment was still significantly below baseline values prior to GnRHa treatment. The pooled random effects model predicted a significant increase from -0.65 at the start of testosterone treatment to -0.12 at followup (p = .0002). Predicted followup BMD-Z was -0.22 SD below baseline but was not significant (p = .12).
Table 4. Differences between BMD z-scores, baseline, start of estrogen treatment, and reference population values.
| Study | Measure | n | vs Baseline | vs Start | vs Reference |
|---|---|---|---|---|---|
| Schagen | spine aBMD | 15 | - | ↑ | - |
| Schagen | hip aBMD | 15 | - | ↑ | - |
| Schagen | body BMD | 15 | ↓ | ↑ | ↓↓↓ |
| Schagen | spine BMAD | 15 | - | ↑ | - |
| Schagen | hip BMAD | 15 | - | ↑ | - |
| Schagen | spine aBMD | 26 | ↓↓ | ~ | ↓↓↓ |
| Schagen | hip aBMD | 26 | - | ~ | ↓↓↓ |
| Schagen | body BMD | 26 | ↓↓↓ | ~ | ↓↓↓ |
| Schagen | spine BMAD | 26 | - | ↑ | - |
| Schagen | hip BMAD | 26 | - | ↑ | ↓↓↓ |
| Klink | spine BMAD | 14 | - | ~ | ↓↓ |
| Klink | spine aBMD | 13 | ↓ | ~ | ↓↓↓ |
| Klink | hip aBMD | 11 | - | ~ | ↓↓ |
| Vlot | hip BMAD | 16 | - (↓) | ~ | ↓↓↓ |
| Vlot | spine BMAD | 15 | ↓↓↓ | ↑ | ↓↓↓ |
| Vlot | hip BMAD | 6 | - | ~ | - |
| Vlot | spine BMAD | 5 | - | ↑ | - |
BMD-Z increased under estrogen treatment in 9 out of 17 cohort-measures but values were still significantly below the reference population in the majority of cohort-measures. In 5 cohort-measures BMD-Z after testosterone treatment was still significantly below baseline values prior to GnRHa treatment. The pooled random effects model showed a non-significant increase of 0.24 from -1.13 at the start of testosterone treatment to -0.89 at followup (p = .14). Modeled followup BMD-Z was -0.29 below baseline and this difference came close to statistical significance (p = .08).
Discussion
GnRHa
BMD under GnRHa treatment stabilises at early adolescent level, declines, or accretes more slowly, when BMD would normally accrete rapidly. Any loss of BMD during adolescence is abnormal ( Ferguson et al., 2019 ). In part due to the typically lower baseline BMD of gender dysphoric adolescents ( Lee et al., 2020 ), an adolescent completing a 1-3 year course of GnRHa could be expected to have BMD around ~1 standard deviation below peers, or about 0.8 SD below baseline.However, care should be taken with interpreting mean values as reported means obscure variation in outcomes. Biggs (2021 ) analysis of the raw values of Joseph et al (2019) highlights how a significant minority of patients had Z-scores below -2, qualifying them as “low for age”. Studies did not track fractures and it is unclear if the treatment induced childhood osteoporosis in any subjects. Most subjects started GnRHa treatment at age 13-15 and were followed up 2-3 years later, limiting conclusions regarding subjects who start treatment earlier and continue for longer. Results suggest that longer treatment duration may lead to greater declines in BMD-Z. All studies lacked controls and lifestyle factors may have impacts on BMD. Standard practice is to counsel patients on the importance of diet and load-bearing exercise ( Oliphant et al., 2018 ). However, the advice to perform load-bearing exercise may not be realistic for gender dysphoric male adolescents who are seeking a female body shape and may lack motivation to undertake a practice they perceive to be at odds with this goal.
Cross sex hormones and treatment withdrawal
BMD can partially or wholly recover to normal levels under subsequent testosterone treatment for the majority of female patients. However, when considering patients of both sexes and the range outcomes following GnRHa treatment, the Bagshaw-Spittlehouse statement that BMD “normalises when GnRH analogues are stopped and either a return to self-produced or cross-sex hormones are started” is not supported. Studies do not demonstrate a recovery of BMD-Z under subsequent estrogen treatment. BMD-Z may stagnate; or improve but may not return to baseline. Schagen et al (2019) reports that three male patients had a femoral neck (hip) BMD-Z less than -2 after three years of estrogen treatment. Three patients also had a spine BMD less than -2 after three years of CSH.Evidence for a recovery of BMD after GnRHA withdrawal and without CSH is lacking and it is unclear how Bagshaw and Spittlehouse support the claim that BMD recovers in this circumstance.
Depo-Provera
Dr Bagshaw’s comparison of GnRHa to depo-provera is also puzzling. Depo-provera is a contraceptive. Consequently, depo-provera treated adolescents will be sexually active and generally older than those prescribed GnRHa for gender dysphoria. GnRHa treatment that starts before and continues through the peak bone mass accretion ages 14-16 may have distinct impacts on BMD compared to a treatment that starts at older ages. Pitts et al. (2012) assessed the impact of depo-provera on BMD and BMD-Z in 83 subjects with a median age of 16.4 at treatment start. In 15 subjects followed up after a further 1.5 years of depo-provera treatment BMD-Z declined significantly (p = .006) at the spine by -0.33 SD, and non-significantly (p = .33) by -0.14 SD at the hip. The former result is less than half the modelled impact of GnRHa treatment of similar duration. However, the small size and older age of subjects make it difficult to compare results between these treatments.Conclusion
The evidence of impacts of GnRHa on bone health in combination with subsequent CSH is limited by the small number of studies, lack of controls and short-term followup. A meta-regression of limited available results shows a significant decline of BMD-Z in GnRHa treated adolescents, a partial or full recovery of BMD-Z in the majority of subsequently testosterone-treated females, and a stagnation or partial recovery of BMD-Z in estrogen-treated males. A literature search was unable to find reported outcomes for gender dysphoric adolescents who suspend hormone treatments altogether post-GnRHa.
It is not clear how Bagshaw, Spittlehouse, and Russell’s literature review arrives at a position of recovery of BMD under both estrogen and endogenous sex hormones post GnRHa treatment withdrawal. A position distinct from both (a) the results reported in this article, and (b), the assessment of other clinicians who work in this area ( Davidge-Pitts and Clarke, 2019 ; Notini et al., 2020 ). Where the evidence review team includes clinicians, statements of professional or reputational conflicts of interest are useful for assessing a potential source of bias. Disappointingly, the claims of recovery of BMD have been repeated in news articles. Patients, their parents, and other clinicians may rely on these sources for medical decision-making. Bagshaw, Spittlehouse, and Russell are advised to clarify this position with supporting evidence.